SAMPLING KIT | SURFACE SAMPLING FOR HAZARDOUS DRUG RESIDUE

Healthcare workers who prepare or administer hazardous drugs or who work in areas where these drugs are used may be exposed to these agents in their workplace.
According to the NAPRA standards the level of hazardous drug contamination should be measured at least every 6 months or more frequently if any major change is made in placement of furniture , aseptic processes, or cleaning and disinfecting practices.
Definition of Hazardous Drugs
Hazardous drugs include antineoplastic drugs used for cancer chemotherapy, antiviral drugs, hormones, some bio-engineered drugs and other miscellaneous drugs. (NIOSH)
USP <800> and NAPRA recommend the surface sampling of antineoplastic residue twice a year.

What are the objectives of performing hazardous drug surface sampling with QUESS-CONTROL ?
Contribute to the assessment of safe practices in the workplace
Evaluate the potential exposure of hazardous drugs to staff
Validate the effectiveness of the decontamination procedure
Assess the quality of the standard operating procedures
Monitor results history with online logs
The marker drugs that can be analyzed for hazardous drug residue fall into one of the five categories defined by BCE Pharma.

fluorouracil

methotrexate

cytarabine, dacarbazine, DOXOrubicin, epirubicin, gemcitabin, melphalan

cyclophosphamide, DOCEtaxel, etoposide, ifosfamide, irinotecan, PACLitaxel, PEMEtrexed, topotecan, vinBLAStine, methotrexate

Platins (CISplatin, CARBOplatin, oxaliplatin)

All hazardous drugs in categories 1 to 4
Flat and non-flat surfaces
Biological safety cabinet, isolator, cart, countertop, floor, shelving unit, etc.
Isolator gloves, i.v bags, vials, door handle, faucets, etc.
A detailed sampling procedure
A form to be completed and returned to BCE Pharma with the samples
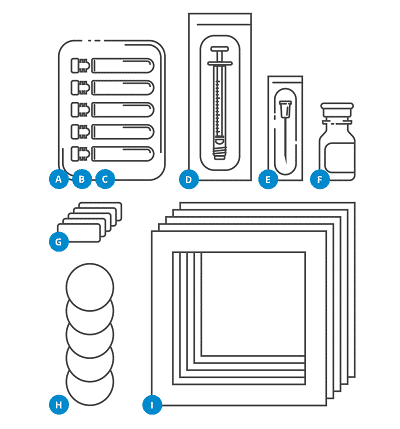
All the items required to perform the sampling by the facility:
A 1 Container
B 10 Glass vials
C 10 Plugs
D 1 Syringe
E 1 Needle
F Sterile water
G Labels
H 10 Filter papers
I 10 Templates
The results will be available on QUESS CONTROL within 3 to 4 weeks following the reception of the samples by the laboratory.
Results are expressed in ng/filter paper
